(Nanowerk Spotlight) Chemotherapeutics generally show a delicate balance between maintaining a high enough dose to kill cancer cells while avoiding a dose so high that it causes severe toxic effects. One of the many promises of nanomedicine is a class of nanoscale drug delivery vehicles that can pinpoint cancer cells and deliver their tumor-killing payload right into cancer cells with high efficiency and no side effects.
As an example of how scientists are approaching this goal, in a recent Nanowerk Spotlight ("Nanorattles are promising as effective drug delivery system") we have provided a first report on in vivo cancer therapy with mesoporous hollow silica nanomaterials. Based on this novel silica nanorattle structure, the Chinese research team further extended their work to fabricate 'all-in-one' multifunctional gold nanoshells on silica nanorattles (GSNs) which combine remote-controlled photothermal therapy with chemotherapy – resulting in a 'magic bullet' to kill cancer cells. The results indicate that a combination of hyperthermia and chemotherapeutic agents is an encouraging approach to optimizing cancer therapy for the synergistic effects are greater than the two individual treatments alone.
Recently, plasmonic nanomaterials as strong near-infrared (NIR) light absorbing agents have provided new opportunities for localized hyperthermia therapy. Although different multifunctional systems based on NIR absorbing nanomaterials have been designed, there have been few attempts to study the synergistic effects of thermo-chemotherapy in one single nanomaterial or further explore their in vivo cancer therapy or systematic toxicity evaluation.
"We first explored the ablation of hepatocellular carcinomas both in vivo and in vitro by the combination of photothermal therapy and chemotherapy using a multifunctional gold nanoshell," Fangqiong Tang, a professor of chemistry at the Laboratory of Controllable Preparation and Application of Nanomaterials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, tells Nanowerk. "Halas's group first applied gold nanoshells coating on solid silica spheres for tumor ablation ("Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance"). Compared to the gold nanoshell on silica solid cores used in Halas's study, the gold nanoshell we use consists of a thin gold nanoshell and a monodispersed mesoporous silica nanorattle core."

A drug-loaded structure comprising a PEGylated (PEG=polyethylene glycol) gold nanoshell on silica nanorattle spheres. (Reprinted with permission from Wiley-VCH Verlag)
Reporting their findings in a recent issue of Angewandte Chemie International Edition ("Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity"), Tang and her team also show that their GSN reduce drug side effects by sustained drug release and provide a new multimodality cancer treatment with higher efficacy and less toxicity than the free drug.
Tang points out the key findings of this new work:
1) Silica nanorattles developed in the Chinese team's lab endow gold nanoshells' many advantages of their unique structure with movable cores and mesoporous shells. They are highly suitable for use in developing intelligent drug delivery systems due to their high thermal, chemical and mechanical stability, large specific surface volume, controllable mesoporous pores, and good biocompatibility.
2) Silica nanorattles' positive changed surface simplifies the gold nanoshell coating process without using any silane coupling agents (e.g.,3-aminopropyltriethoxysilane) modification step like other reports.
3) The combination of hyperthermia and chemotherapeutic agents is an encouraging approach which can result in synergistic effects that are greater than the two treatments alone. Gold nanorods have been reported producing heat to augment the toxicity of chemotherapeutic agents. But by simply mixing gold nanorods and chemotherapeutic agents, the synergistic effects of thermo-chemotherapy are difficult to realize in vivo because co-delivery of chemotherapeutic agents together with gold nanorods-induced hyperthermia sources to the target tissues is still challenging. GSNs, on the other hand, have a tunable optical property as NIR light absorbing agents and a high payload. They can co-deliver chemotherapeutic agents together with hyperthermia sources to the target tissues.
4) Even though different multifunctional systems based on NIR absorbing nanomaterials have been designed, many parameters of these systems were only assessed in vitro in cellular systems, while no in vivo study of thermo-chemotherapy effect of plasmonic nanomaterials based on gold nanoshells have been investigated.
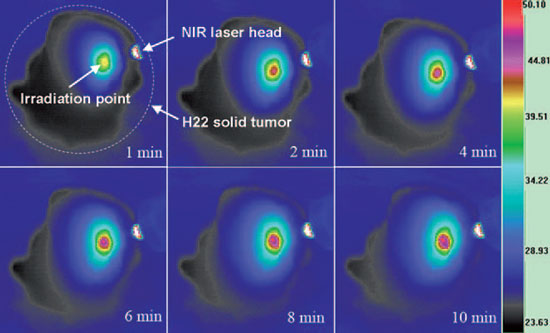
Infrared thermal images of an excised pGSNs-injected H22 solid tumor sample at different time points under NIR laser irradiation. The colored bar represents the relative temperature values in °C. The dashed circle indicates the H22 solid tumor. (Reprinted with permission from Wiley-VCH Verlag)
The nanostructures used by Tang and her team consist of a thin gold nanoshell and a monodispersed mesoporous silica nanorattle core. This kind of plasmonic nanomaterial exhibits a strong optical extinction at near-infrared wavelengths (700–850nm) owing to the localized surface plasmon resonance of their free electrons upon excitation by an electromagnetic field. So they can absorb the NIR light in resonance and transfer the thermal energy to the surrounding medium or tissue.
GSNs are a promising building block with many biomedical applications, such as biological imaging, thermal ablative cancer therapy and immunoassays. Due to the specific silica nanorattle core, GSNs are also promising as a versatile and multifunctional drug delivery platform for their high-payload delivery of various drugs into their targets. The payloads could be small drug molecules or large bimolecules, such as proteins, DNA, RNA and other therapeutic agents.
Going forward, the researchers still need to optimize the photothermal therapy and chemotherapy process and quantify the tissue damage.
"Moreover" says Tang, "the exact mechanism of combining the two therapies is still not completely understood. Whether increasing the temperature also induces immunological changes – for instance, the release of heat shock proteins and subsequently maybe an improved immune recognition – is totally unknown and worth investigating."
Another objective the team will attempt to achieve is to improve advanced cancer therapeutic efficacy by combining the GSNs with radiation therapy. Many cancer patients are often diagnosed at an advanced stage, at which time the tumors are often unresectable, more aggressive and less responsive to standard treatments, including radiation. A synergistic therapy, combining chemo- and photothermal treatments with radiotherapy, might therefore result in a promising alternative strategy.
(Source: Nanowerk . By Michael Berger.)

