Recently, Laboratory of Supramolecular Photochemistry of TIPC CAS developed the first ratiometric fluorescent sensor for highly selective detection of glutathione (GSH) in living cells, published on Journal of the American Chemical Society.
The researchers reported a BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine (Cys) and homocysteine (Hcy). The chlorine of the monochlorinated BODIPY can be rapidly replaced by thiolate. The amino groups of Cys/Hcy, but not of GSH further replace the thiolate to form amino-substituted BODIPY. The significantly different photophysical properties of sulfur and amino substituted BODIPY enable the discrimination of GSH over Cys/Hcy. The sensor was applied for detection of GSH in living cells.
Biological thiols, including cystein (Cys), homocystein (Hcy) and glutathione (GSH), play crucial roles in maintaining biological systems. As the most abundant cellular thiol, GSH is an essential endogenous antioxidant which can defense against toxins and free radicals. However, most of the known fluorescent methods cannot discriminate between SH-containing molecules because of their similarity in structure and reactivity. Thus, it is still a challenge to develop fluorescent sensors for selective detection of GSH. The unique and interesting reaction mechanism may inspire the exploration of new systems for the selective detection of biothiols.
This work is support from the National Key Basic Research Program of China, the National Natural Science Foundation of China, and the Chinese Academy of Sciences.
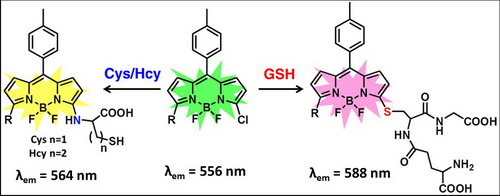
Ratiometric fluorescent sensor for selective detection of GSH
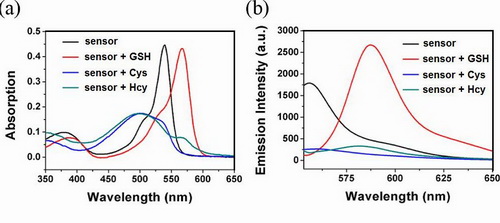
(a) Absorption and (b) emission spectra of fluorescent sensor before and after the addition of GSH, Cys and Hcy.

