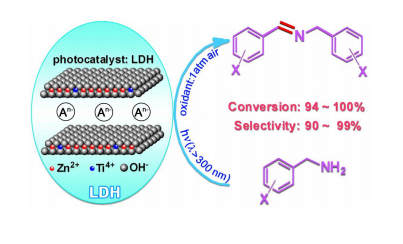
 Imine derivatives represent important intermediates for the synthesis of dyes, pharmaceuticals, agrochemicals and agricultural chemicals.Over the past decades, a great deal of efforts have been made to develop mild and practical oxidation reactions for imines preparation. Whereas the traditional condensation of aldehydes and amines has been well-established, direct oxidation of amines to imines is of intense current interest. In particular, the use of light and molecular oxygen to trigger the conversion of amines to imines has aroused much attention. For instance, Che et al.reported that a variety of secondary aromatic amines could be oxidized to imines in 90% to >99% yields using singlet oxygen generated from oxygen and a porphyrin photosensitizer. Wang and Blechert presented the aerobic oxidation of amines into imines in excellent yields by a mesoporous graphite carbon nitrile (mpg-C3N4) photocatalyst. Zhao et al.found a series of aromatic amines being transformed into the corresponding imines under light irradiation, air and TiO2 in acetonitrile. Despite of the fact that considerable efficient methods have been developed for the oxidation of secondary amines to imines, little attention had been given to the oxidation of primary amines until recently,probably because the generated imines are intermediate products that are easily dehydrogenated to nitriles. Scientists of TIPC,CAS reported the first application of layered double hydroxide as a photocatalyst in the transformation of primary aromatic amines to their corresponding imines with high efficiency and selectivity by using oxygen in air atmosphere as a terminal oxidant under light irradiation. Chem. Commun., 2014 |

