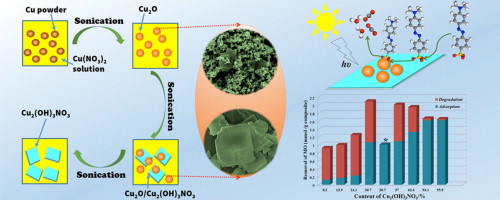
Composites having both maximized adsorption capability and high photocatalytic activity are very promising for pollutant removal by combining adsorption and photocatalysis. In this work, we report a facile sonochemical synthesis of Cu2O(nanoparticles)/Cu2(OH)3NO3(sheets) composites with tunable composition using only copper powders and Cu(NO3)2 as precursors. The composites can be controlled from almost pure Cu2O (98.1%) to pure Cu2(OH)3NO3 (95.9%) through adjusting the initial ratio of Cu2+:Cu0 and sonication time. Controlled experiments show that Cu2O nanoparticles are formed first viathe redox reaction between Cu2+ and Cu0, after that Cu2O are converted to Cu2(OH)3NO3. Cu2(OH)3NO3synthesized using the sonochemical method exhibits outstanding adsorption capability toward organic dyes, subsequently Cu2O can degrade them quickly via photocatalysis, so the Cu2O/Cu2(OH)3NO3composites are very efficient to remove pollutant due to the synergetic effect of adsorption and photocatalysis. In particular, composite with 30.6% of Cu2(OH)3NO3 and 69.4% of Cu2O shows the highest removal capability and good stability. Applied Catalysis B: Environmental, 2015 |

