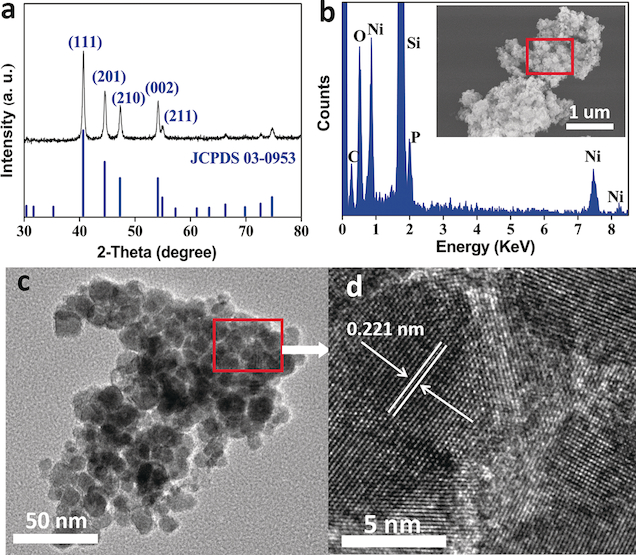
a) XRD pattern and b) EDX results corresponding to a selected scanning electron microscope image (inset); c) TEM and d) HRTEM images of obtained Ni2P NPs. Ammonia–borane (AB) is a promising chemical hydrogen-storage material. However, the development of real-time, efficient, controllable, and safe methods for hydrogen release under mild conditions is a challenge in the large-scale use of hydrogen as a long-term solution for future energy security. A new class of low-cost catalytic system is presented that uses nanostructured Ni2P as catalyst, which exhibits excellent catalytic activity and high sustainability toward hydrolysis of ammonia–borane with the initial turnover frequency of 40.4 mol(H2) mol(Ni2P)−1 min−1 under air atmosphere and at ambient temperature. This value is higher than those reported for noble-metal-free catalysts, and the obtained Arrhenius activation energy (Ea=44.6 kJ mol−1) for the hydrolysis reaction is comparable to Ru-based bimetallic catalysts. A clearly mechanistic analysis of the hydrolytic reaction of AB based on experimental results and a density functional theory calculation is presented. Angewandte Chemie, 2015 |

