In this paper, (vapour + liquid) equilibrium (VLE) for the {1,1-difluoroethane (R152a) + 1,1,1,3,3-pentafluoropropane (R245fa)} system was determined by a static-analytical method at T = (323.150 to 353.150) K. Values of the VLE were correlated by the Peng–Robison equation of state (PR EoS) using two different models, the van der Waals (vdWs) mixing rule and the Huron–Vidal (HV) mixing rule involving the non-random two-liquid (NRTL) activity coefficient model. The correlated results show good agreement with the experimental values. For the two models, the maximum average absolute deviations of the vapour phase mole fraction are 0.0034 and 0.0035, respectively. The Journal of Chemical Thermodynamics, 2015 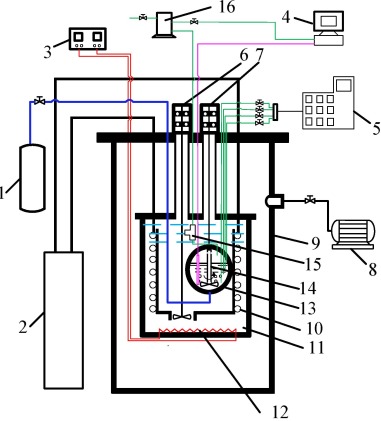
A schematic of the experimental apparatus. 1. feed system; 2. digital controller; 3. digital controller; 4. temperature and pressure indicator; 5. GC; 6,7. motors; 8. vacuum pump; 9. vacuum vessel; 10. cooling coil; 11. isothermal liquid bath; 12. electric heater; 13.equilibrium cell; 14. stirrers; 15. pressure transducer; 16. N2-filled system. |

