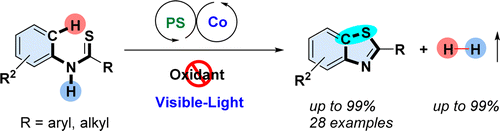
An external oxidant-free oxidative coupling for aromatic C–H thiolation by visible-light photoredox cobalt-catalysis has been developed. Various substrates could afford benzothiazoles in good to excellent yields, and only H2 is generated as a side product. When catalytic TBAOH was used as the base, not only 2-aryl but also 2-alkylbenzothiazoles could be obtained through this novel dehydrogenative coupling reaction. This method could be scaled up and applied to the synthesis of biologically active molecules bearing benzothiazole structural scaffolds (potent antitumor agents). Furthermore, the unexpected oxidation byproduct amides, which are often generated in oxidative cyclization of thiobenzanilides, can be completely avoided. Mechanistic studies showed that the H2 originates from the substrates. The kinetic studies indicate that the interaction between the cobalt catalyst and proton might be involved in the rate-limiting process. J. Am. Chem. Soc., 2015 |

