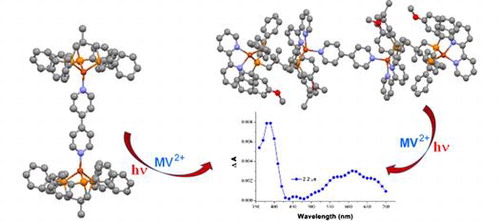
Three mono-, bi- and tetranuclear copper(I) complexes, [Cu(phen)(triphos-O)]BF4(1) (phen = 1,10-phenanthroline, triphos = 1,1,1-tris(diphenylphosphinomethyl)ethane), [Cu2(bipy)(triphos)2](BF4)2 (2) (bipy = 4,4′-bipyridine), and [Cu4(MeOC^N^N)4(triphos)2(bipy)](BF4)4 (3) (MeOC^N^N = 6-(4-methoxyphenyl)-2,2′-bipyridine), have been synthesized and characterized by NMR spectroscopy, electrospray ionization, and matrix-assisted laser desorption ionization time-of-flight mass spectrometries, elemental analysis, and X-ray crystal analysis. The crystal structure investigation revealed the copper ions of the complexes have pseudo-tetrahedral coordination geometry. The electronic absorption spectra of 1, 2, and 3 contain low-energy bands at 350–500 and 400–650 nm, which are assigned to d(Cu) → π*(phen or bipy) and a mixture of d(Cu) → π*(MeOC^N^N) and d(Cu) → π*(bipy) transitions, respectively. Complex 2 displays a strong, long-lived solid-state emission with a maximum at 555 nm and lifetime of 13.6 μs at room temperature. Photoinduced electron-transfer properties of 2 and 3 involving nanosecond time-resolved absorption spectroscopy and electron spin resonance techniques were studied. Journal of Coordination Chemistry, 2015 |

