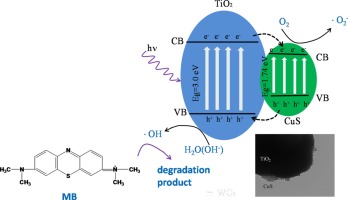
CuS nanoflowers, fabricated by an element-direct-reaction route using copper and sulfur powder, were loaded on rutile TiO2 (CuS/TiO2) at low temperature. CuS/TiO2 composites were utilized as the photocatalysts for the degradation of Methylene Blue (MB) and 4-chlorophenol (4-CP). X-ray diffraction (XRD), UV Raman spectroscopy, transmission electron microscopy (TEM), XPS, and UV-visible diffuse reflectance spectra were used to characterize the crystalline phase, morphology, particle size, and the optical properties of CuS/TiO2 samples. It is found that CuS/TiO2 photocatalyst, which CuS are loaded on the surface of rutile TiO2, exhibited enhanced photocatalytic degradation of MB (or 4-CP) than TiO2 or CuS. This indicates that CuS can enhance effectively the photocatalytic activity of rutile TiO2 by forming heterojunction between CuS and rutile TiO2, which is confirmed by photoluminescence (PL) spectra and TEM. Moreover, CuS content has a significant influence on photocatalytic activity and 2 wt% CuS/TiO2 showed the maximum photocatalytic activity for degradation of MB. Applied Surface Science Available online 23 February 2016 |

