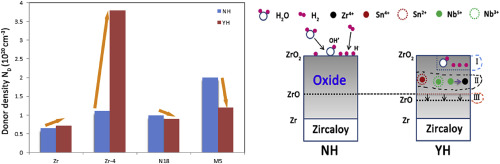
Zircaloys, rooted in their inherent passivity, are widely used as the cladding materials for light water reactors (LWRs) due to their desirable corrosion resistance against high temperature and high pressure water. The hydrogen permeation behavior on zircaloy with passive film, however, is rarely understood so far. In this work, the gaseous hydrogen permeation characteristic mainly on the defect evolution of nanoscale passive films of nanoscale passive films formed hydrothermally on different types of zirconium alloys (Zirconium, Zircaloy-4, N18 and M5) was explored and compared. Surface analytical techniques were implemented to evaluate the hydrogen permeation characteristic. The AES depth profiles showed that the as-prepared oxides were hundreds of nanometers in thickness and inward diffusion occurred upon hydrogen exposure. Electrochemical measurements suggested that the oxides formed on different types of zircaloys were of similar phase structures of monoclinic zirconia but with different oxygen vacancy concentrations. XPS results comparison confirmed that niobium enhances the hydrogen resistance of the oxides, yet tin causes degradation of the oxides in the reductive environment. In view of these analyses, the involving mechanism was first proposed and discussed on the basis of point defect reactions. International Journal of Hydrogen Energy Available online 24 March 2016 |

