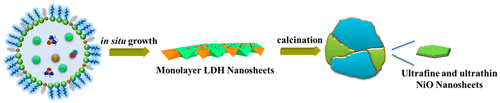
Faceted NiO nanoparticles preferentially exposing high surface energy planes demand attention due to their excellent electrocatalytic properties. However, the activity of faceted NiO nanoparticles generally remains suboptimal due to their large lateral size and thickness, which severely limits the availability of coordinatively unsaturated active reactive edge and corner sites. Here, ultrafine NiO nanosheets with a platelet size of ~4.0 nm and thickness (~1.1 nm) stabilized by TiO2 were successfully prepared by calcination of a monolayer layered double hydroxide precursor. The ultrafine NiO nanosheets displayed outstanding performance in electrochemical water oxidation due to a high proportion of reactive NiO {110} facets, intrinsic Ni3+ and Ti3+ sites, and abundant interfaces, which act synergistically to promote H2O adsorption and facilitate charge-transfer. J. Am. Chem. Soc., Article ASAP DOI: 10.1021/jacs.6b01606 Publication Date (Web): May 09, 2016 |

