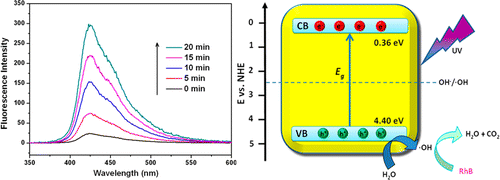
 Nonbonding layer-structured Y(IO3)3 was successfully prepared by a simple hydrothermal route and investigated as a novel photocatalyst for the first time. Its crystal structure was characterized by X-ray diffraction, high-resolution transmission electron microscopy, and scanning electron microscopy. The optical absorption edge and band gap of Y(IO3)3 have been determined by UV–vis diffuse reflectance spectra. Theoretical calculations of the electronic structure of Y(IO3)3 confirmed its direct optical transition property near the absorption edge region, and the orbital components of the conduction band and valence band (VB) were also analyzed. The photocatalytic performance of Y(IO3)3 was evaluated by photooxidative decomposition of rhodamine B under ultraviolet light irradiation. It demonstrated that Y(IO3)3 exhibits highly efficient photocatalytic activity, which is much better than those of commercial TiO2 (P25) and important UV photocatalysts BiOCl and BiIO4. The origin of the excellent photocatalytic performance of Y(IO3)3 was investigated by electron spin resonance and terephthalic acid photoluminescence techniques. The results revealed that the highly strong photooxidation ability that resulted from its very positive VB position should be responsible for the excellent photocatalytic performance. Inorganic Chemistry, 2014 |

