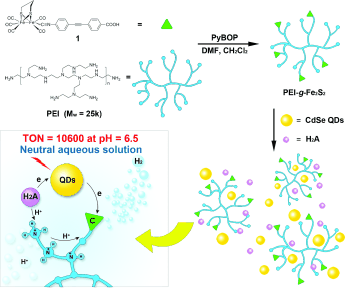
Nature uses hydrogenase enzyme to catalyze proton reduction at pH 7 with overpotentials and catalytic efficiencies that rival platinum electrodes. Over the past several years, [FeFe]-hydrogenase ([FeFe]-H2ase) mimics have been demonstrated to be effective catalysts for light-driven H2 evolution. However, it remains a significant challenge to realize H2 production by such an artificial photosynthetic system in neutral aqueous solution. Herein, we report a new system for photocatalytic H2 evolution working in a broad pH range, especially under neutral conditions. This unique system is consisted of branched polyethylenimine (PEI)-grafted [FeFe]-H2ase mimic (PEI-g-Fe2S2), MPA-CdSe quantum dots (MPA=mercaptopropionic acid), and ascorbic acid (H2A) in water. Due to the secondary coordination sphere of PEI, which has high buffering capacity and stabilizing ability, the system is able to produce H2 under visible-light irradiation with turnover number of 10 600 based on the Fe2S2 active site in PEI-g-Fe2S2. The stability and activity are much better than that of the same system under acidic or basic conditions and they are, to the best of our knowledge, the highest known to date for photocatalytic H2 evolution from a [FeFe]-H2ase mimic in neutral aqueous solution. Chemistry - A European Journal, 2015 |

