When it comes to hydrogen production, people think of the electrolysis or photolysis of water. However, in these processes, the energy efficiency stays low, and the production capacity could be significantly enhanced.
Among the many technological strategies ever tried, the Al-H2O reaction is a rather favored way to produce hydrogen, although it is always hindered by a layer of passive film on Al surface. Recently, a class of gallium-based room-temperature liquid metal (RTLM) was found to effectively activate the Al-water reaction, which is expected to achieve real-time and on-demand hydrogen generation.
The underlying mechanism of RTLM triggered Al-water reaction relies on three terms. According to the Rehbinder effect, the liquid gallium could break the oxide film on Al surface, and penetrate into Al bulk, forming a solid solution. As we know, the reactivity of metal depends on its electrode potential in the electrolyte solution. Part of indium and tin exist in the form of intermetallic compounds on the Al grain boundaries, which causes the negative shift of electrode potential of Al, thus activating the hydrolysis reaction of Al. Last but not the least, numerous micro corrosion cells forms between Al and RTLM due to their electrode potential difference, which accelerates the corrosion of Al anode, facilitating the hydrogen liberation. With the development of computational materials science and modern material analysis and testing technology, more reaction mechanisms and details are revealed on the atomic scale.
In order to regulate the hydrogen release rate, it is of significance to investigate the influencing factors that dominate the reaction rate. Former researches have proven that the RTLM composition, reaction temperature, electrolyte solution, and substrate effectively influence the Al-water reaction, which provides available approaches to quantitatively adjust the reaction and optimize the hydrogen production performance.
Additionally, gallium-based liquid metal alloys inherit considerable excellent properties, with surface tension nine times larger than water, kinematic viscosity approximately one-fourth of water, nontoxicity compared to mercury, and favorable conductivity. Based on these characters, RTLM containing Al presents amusing biomimetic behaviors in solution under the synergistic effect of hydrogen release and surface tension, like running, loading, oscillating, deformation, and jumping (with nickel). These phenomena provide a new insight into the application of hydrogen from Al-H2O reaction and offer a new thought for developing future soft machines.

Fig. 1. Fantastic phenomena caused by hydrogen generation from liquid metal activated metal-water reaction. a. Self-propulsion liquid metal motors. b. Self-propelled liquid metal motors steered by external fields for drug delivery. c. Liquid metal machine triggered violin-like wire oscillator. d. Liquid metal amoeba with spontaneous pseudopodia formation and motion capability.
Above all, RTLM-triggered Al-H2O reactions are a promising scheme to generate hydrogen from water in real-time and on-demand with minimal external energy costing and without producing any contaminants. However, there are still challenges in terms of theory and techniques. Computational materials science and microstructure test technology should keep pace to illuminate the mechanism of the RTLM activated Al-H2O reaction. Meanwhile, more appropriate techniques need to be developed to guarantee the precise control of hydrogen production, the recovery of RTLM and Al(OH)3, and mass production of hydrogen by this approach.
In the long-term, the application of hydrogen production from RTLM activated Al-H2O reaction in both the energy and machine fields is inspiring, with outstanding safety, environmental protection, cyclic performance, and flexible controllability. The system thus established can serve as a preferred power source for practical vehicles in the area of ground, sea, and air. There is no doubt that the RTLM triggered Al-H2O reaction would bring a renaissance of hydrogen energy. Nevertheless, industrial application of this method to produce clean hydrogen is a marathon, which is accelerating. It is certain that “Hy-time” is coming closer and closer.
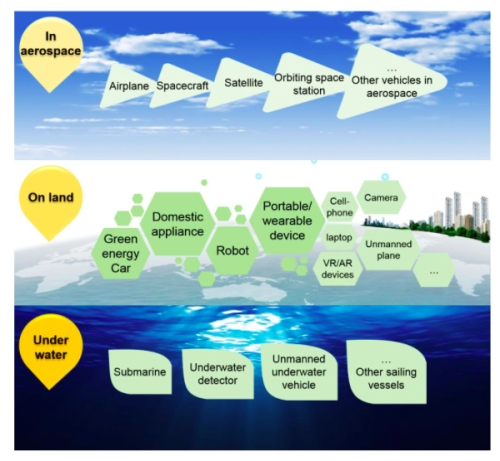
Fig. 2. Application examples of RTLM triggered Al-H2O reaction for hydrogen generation.
This work is described in the article titled Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature, recently published in the Renewable & Sustainable Energy Reviews. The work was conducted at the Key Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences by Shuo Xu, Xi Zhao, Jing Liu. (ScienceTrends)
This feature was originally published on the Science Trends (s (https://sciencetrends.com/liquid-metal-activated-al-water-reaction-a-new-approach-leading-to-hy-time/). Sci Science Trends is a leading source of science news and analysis on everything from climate change to cancer research, and it is a free platform for scientists to directly share their research with a large and global audience.
NEWS