RNA interference (RNAi) provides a promising strategy for treating hereditary disease, cancer, HIV-1 and so on. Due to the negative charge of naked nucleic acids, a safe and efficient vector becomes the primary challenge in RNAi. Viral vectors such as adeno- and retroviruses have covered most gene vectors in clinical trials, showing high gene transfection efficacy, but their inherent immunogenicity, pathogenicity and probable chromosomal integration become major barriers for their clinical trials. Non-viral gene vectors such as liposomes and cationic polymers show low immunogenicity and can achieve multifunctional assemblies through intelligent design, but the gene transfection efficiency is still not comparable to that of viral vectors.
Recently, inspired by the high efficiency of viruses and the safety of non-viruses, a 1D rod-like gene delivery vector based on tobacco mosaic virus (TMV) was reported by Ye Tian and Zhongwei Niu group from Technical Institute of Physics and Chemistry, CAS. Specifically, cell-penetrating peptide TAT was decorated to the exterior surface of TMV to provide endosomal escape property, and small interfering RNA (siRNA) was loaded onto the TMV-TAT vector through electrostatic interactions. The obtained gene delivery system (siRNA@TMV-TAT) showed effective green fluorescent protein (GFP) silencing capacity, in both in vitro and in vivo assessment. Compared with the commercial transfection reagents Lipofectamine 2000 and PEI25k, TMV-TAT vectors showed much higher gene transfer efficiency at a similar safety level.
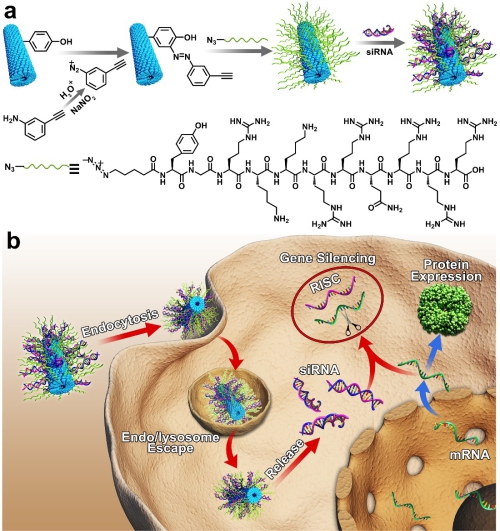
(a) Schematic illustration for the design of TAT-decorated TMV and siRNA loading. (b) Schematic illustration of the gene silencing process. (Image by TIAN Ye)
This work combines the high efficiency of viral vectors and the safety of non-viral vectors and may provide a promising strategy for RNA interference technology. This work entitled “Integration of Cell-Penetrating Peptides with Rod-like Bionanoparticles: Virus-Inspired Gene Silencing Technology” was recently published in Nano Letters (DOI: 10.1021/acs.nanolett.8b01805).
This work was supported by the Beijing Natural Science Foundation, the National Key R&D Program of China, the National Natural Science Foundation of China, the Youth Innovation Promotion Association of the Chinese Academy of Sciences and the Presidential Foundation of Technical Institute of Physics and Chemistry.
Link: https://pubs.acs.org/doi/10.1021/acs.nanolett.8b01805
Email:tiany@mail.ipc.ac.cn
NEWS