Scientists have identified that intrinsic carbon defects in carbon materials serve as active sites for CO2 electroreduction. This work was published in Advanced Materials.
In a recent pioneering breakthrough, a research team led by Prof. ZHANG Tierui (Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing) discovered the significant contribution of intrinsic carbon defects to electrocatalytic CO2 reduction reaction in carbon-based catalysts was revealed by experiments and DFT for the first time. Heteroatom-doped carbon catalysts are currently attracting enormous attention due to their excellent performance for the electrocatalytic carbon dioxide reduction reaction (ECRR). However, the origin of the high catalytic activities of doped-carbon materials remains obscure with the role of intrinsic carbon defects in promoting the ECRR receiving little attention despite the abundance of carbon defects in all carbon-based catalytic materials. Zhang’s team found a positive correlation between the ECRR performance of carbon-based catalysts and the content of intrinsic carbon defects contained within these catalysts. Further, defective porous carbon catalysts containing no active heteroatom dopants also show excellent catalytic performance for ECRR. C K-edge near edge X-ray absorption fine structure measurements and density functional theory calculations revealed that sp2 defects (octagonal and pentagonal) rather than edge defects are key to the excellent ECRR activity of defective porous carbon catalysts.
This work introduces a new paradigm in the development of electrocatalysts for ECRR by identifying the critical role of carbon defects as active sites. Results guide the development of new and improved electrocatalysts for CO2 reduction.
This work is financially supported by the National Key Projects for Fundamental Research and Development of China (2017YFA0206904, 2017YFA0206900, 2016YFB0600901), the National Natural Science Foundation of China (51825205, 51772305, 51572270, 21871279, U1662118), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB17000000), the Beijing Natural Science Foundation (2182078), the Beijing Municipal Science and Technology Project (Z181100005118007), the Royal Society-Newton Advanced Fellowship (NA170422), the International Partnership Program of Chinese Academy of Sciences (GJHZ1819), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. GINW acknowledges funding support from the University of Auckland Faculty Research Development Fund, the Energy Education Trust of New Zealand and the Ministry of Business Innovation and Employment (MBIE) Catalyst Fund, Contract MAUX1609: "Disruptive Technologies from Metal-Organic Frameworks".
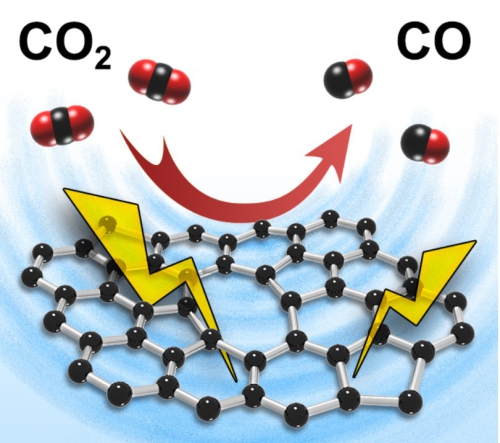
Figure. Electrocatalytic CO2 reduction reaction on intrinsic carbon defects in carbon-based catalysts.
https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201808276
Email: tierui@mail.ipc.ac.cn
NEWS