Bacterial infections pose a threat to human health. Now, with increasing antibiotic resistance, such infections may again ravage humanity as they did in the pre-antibiotic era. Scientists are thus seeking new, non-antibiotic means to combat bacterial infection.
One promising strategy is photodynamic inactivation (PDI). It uses photosensitizers to generate reactive oxygen species (ROS) that damage bioactive substances in the cell membrane, thus causing irreversible bacterial death in the presence of light and O2. Unfortunately, ROS has a short half-life and reaction radius. As a result, a big challenge for PDI is how to enhance membrane intercalation.
Recently, researchers from the Technical Institute of Physics and Chemistry (TIPC) of the Chinese Academy of Sciences, Shanghai Jiao Tong University and the University of Utah reported their work on achieving enhanced membrane intercalation.
In this work, the scientists arranged for a peptide-decorated cell-penetrating virus coat protein (TAT-TMVCP) and an organoplatinum metallacycle (TPE-Pt-MC), which acts as a photosynthesizer with aggregation-induced emission, to self-assemble through electrostatic interaction.
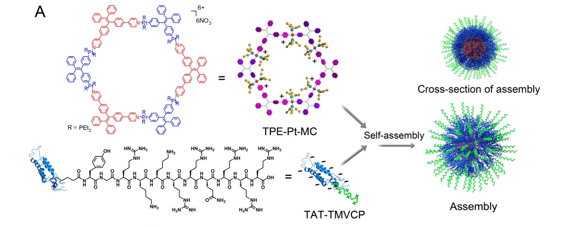
Figure (A) Schematic illustration of the self-assembly of TPE-Pt-MC and TAT-TMVCP
In the “new” assembly, the photosensitizer provides ROS-generation capacity. The peptide exposed on the surface provides membrane-intercalating capacity.

Figure (B) Antibacterial mechanism of the assembly
The researchers discovered that the assembly achieved significantly enhanced PDI efficiency against E. coli and S. aureus, especially against gram-negative E. coli. The assembly decreased E. coli‘s survival rate from 55% in the dark to nearly 0% upon light irradiation.
This study has wide implications, ranging from improving PDI efficiency to generating multifunctional nanomaterials.
The results, entitled “Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated virus coat protein,” were published in PNAS on November 4 2019.
This research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the National Institutes of Health of the U.S., and the Beijing Municipal Natural Science Foundation, among others.
Ref: https://doi.org/10.1073/pnas.1911869116
NEWS