Recently, Prof. WANG Naixing’s research group from Technical Institute of Physics and Chemistry, Chinese Academy of Sciences (TIPC, CAS) found azaarenes and ethers can directly undergo a coupling reaction under the catalysis of rare earth catalyst yttrium triflate, by using di-tert-butyl peroxide as a radical initiator. The direct coupling reaction belongs to Cross-Dehydrogenative-Coupling (CDC) reaction to achieve functionalization of C(sp3)-H bond. This protocol has the advantages of good atom economy and strong selectivity.
In the past years, Prof. WANG’s group has made a series of progresses in the functionalization of unactivated C(sp3)-H bond. Bifunctionalizaions of styrene with alcohols, ketones, nitriles, and ethers were developed and a number of high IF articles were reported in Org. Lett and other journals. Synthesis (Synthesis, 2019, 51, 4531) even reviewed the works and summarized into a general reaction formula (“Wang’s reaction”).
In this latest reaction study, it is found the rare earth catalyst has a better catalytic effect than other Lewis acid catalysts, such as Cu(OAc)2. Rare earth yttrium ions belong to hard Lewis acids and have a strong coordination ability with nitrogen or oxygen atoms. The azaarene and ether can be coordinated with yttrium ions to form an active transition state. It makes the reaction easy to proceed.
This reaction features a broad substrate scope. Azaarenes can react with ethers and thioethers to furnish the C(sp3)-H bond functionalized product. In addition to chain ethers, cyclic ethers can also effectively undergo this C(sp3)-H bond functionalization reaction. The 31 products obtained were all characterized by Nuclear Magnetic Resonance Spectrometry (NMR), and High-Resolution Mass spectrometry (HRMS).
Prof. WANG’s group conducted mechanistic studies to have further insight into the reaction mechanism. They added radical scavenger to the reaction system under the standard conditions. It was found that the transformation was completely inhibited, and the adduct of radical scavenger and radical generated was detected by HRMS. The results suggested that the present reaction should involve a free radical process. In the kinetics isotope experiments, it is successfully verified that α-C(sp3)-H bond cleavage of ether is the rate-determining step.
This work was published in Organic Letters (Org. Lett. 2019, 21, 7450-7454).The first author WU Yuehua was a PhD student in WANG's group (now is teaching at a university in Guangzhou).
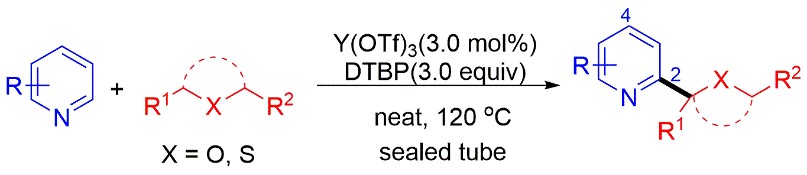
Figure 1. Coupling Reaction of Azaarenes and Ethers

Figure 2. WANG’s Reaction
https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0039-1690674
https://pubs.acs.org/doi/10.1021/acs.orglett.9b02763
NEWS