Chinese researchers recently prepared a series of ultrafine cuprous oxide (Cu2O) with a particle size of less than 3 nm using the topological transformation properties of layered double hydroxide (LDH). It is applied to the visible light-driven reduction of nitrogen (N2) to ammonia (NH3) for the first time.
This work, published online in Angew. Chem. Int. Ed., was directed by Prof. ZHANG Tierui from Technical Institute of Physics and Chemistry (TIPC) of the Chinese Academy of Sciences.
Catalytic reduction of N2 to NH3 is one of the most important chemical industrial processes in human society, but causes high energy consumption and high carbon emissions. Therefore, it is urgent to find a green and sustainable new way of NH3 synthesis.
In recent years, photocatalytic technology has proven to be a promosing green pathway toward NH3 synthesis by N2 reduction at ambient temperature and pressure. The development of efficient and stable photocatalysts to promote solar light absorption, extend carrier life and improve the kinetics of nitrogen fixation is an important prerequisite for achieving clean solar synthesis of NH3.
Cu2O is a low-cost and easily accessible semiconductor material with good visible light absorption properties and appropriate energy band structure for the N2 reduction and water oxidation reaction, but has not yet been exploited in the field of photocatalytic N2 reduction. Bulk Cu2O with large particle size usually demonstrates unsatisfactory photocatalytic properties due to the severe recombination of electrons and holes and limited exposure of surface sites. The design and synthesis of ultrafine Cu2O is expected to address the above-mentioned issues, but it is still very challenging to synthesize ultrafine Cu2O at ambient conditions, which greatly hinders its further catalytic application.
In this work, the researchers found that both the confinement effect of the LDH and the control of reduction process contribute to the synthesis of sub-3 nm Cu2O with highly tunable size and loading at room temperature and pressure. The as-prepared sub-3 nm ultrafine Cu2O possesses abundant electron trapping states and highly exposed surface active sites, which greatly promotes the separation/transport of photo-generated carriers and the adsorption/activation of N2 molecules, thus delivering excellent the photocatalytic performance of N2 reduction to NH3.
This work was supported by the National Science Foundation of China, and the International Partnership Program of Chinese Academy of Sciences (GJHZ1819).
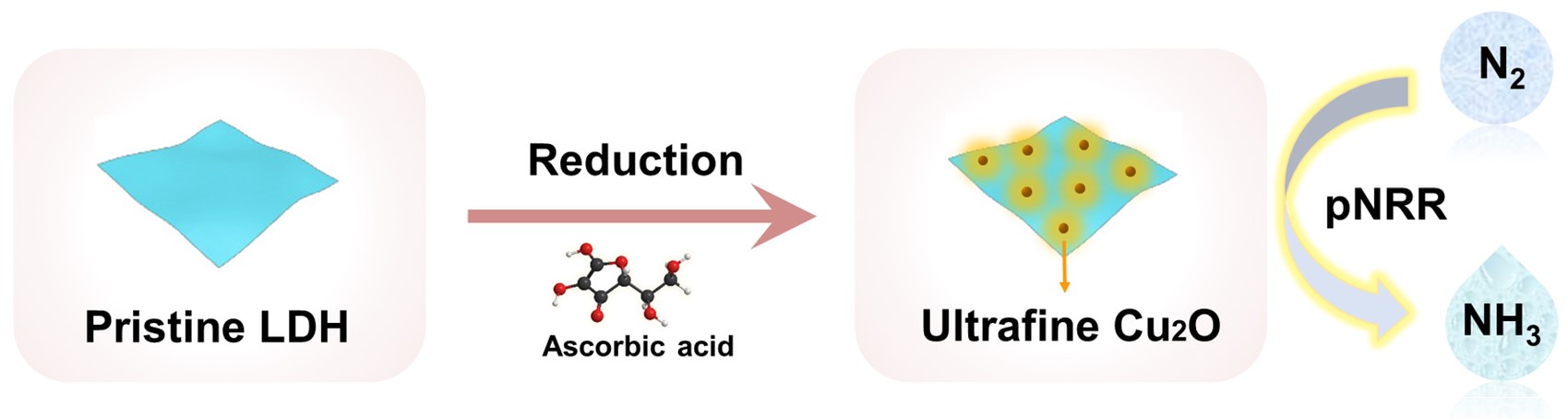
Synthetic strategy used to prepare LDH-supported sub-3 nm ultrafine Cu2O
https://www.doi.org/10.1002/anie.202013594
NEWS